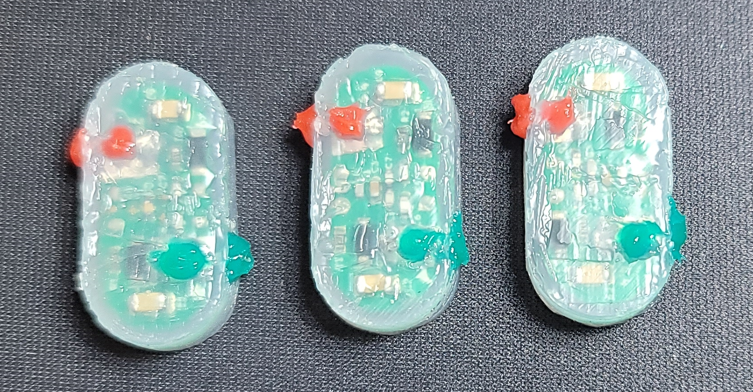
IPG, Receiver & Adapter
Investigational device. Availability subject to applicable regulatory approvals.
IPG and receiver options expanding Injectrode uses
To provide future patients and physicians with options, Neuronoff is developing a “Handshake” adapter that enables the connection of Injectrodes to an IPG (Neuronoff / MDT / BSX / ABT / NVRO / Biotronik) or Receiver (Neuronoff / Nalu / Curonix).
Future patients will have a 90-day period to experience neuromodulation without surgery: An Injectrode is placed by an 18 gauge needle and powered by an External Pulse Generator (EPG), worn only during treatment times. There are no protruding wires, minimal risk for scars and the removal is as straightforward as the placement. This Try Lead phase lowers the barriers to a drug-free pain treatment.
At the end of this Try Lead phase, the patient will have three options.
They may want to continue using only the EPG to drive the pain relief - or they may prefer an implantable pulse generator (IPG) featuring a battery, or as a third option, an implantable resonance-coupled driver circuit (Receiver) that does not need a battery. The Handshake adapter allows the connection of one or more Injectrodes to an IPG or Receiver.
Figure 1 An elegant Try Lead phase will allow future patients to try out neuromodulation without surgery. If they would like to continue the treatment beyond 90 days, chronic options include a Handshake adapter that enables the connection to traditional IPGs or Receiver circuits, some of which may be provided by a partner company.
Wirelessly powered neuromodulation with no implanted battery
While most patients may prefer an EPG to power the implanted Injectrode, some patients may elect for a fully implanted Receiver as a type of pulse generator that derives its energy from an externally worn power source in the size and shape of a watch. Without an implanted battery, Receivers do not require surgeries every 5-7 years for battery replacement. Neuronoff has developed a first version that is undergoing chronic preclinical testing.
Figure 2 Genicular nerve example: following a 90 day Try Lead phase, a Receiver circuit is connected to the already implanted Injectrode and placed into the subcutaneous tissue. An external driver the size of a wristwatch powers the Receiver inductively.
Design with minimal size in mind
Neuronoff is testing resonance-coupled Receiver circuits in chronic preclinical models at this time. Single- and multi-channel Receivers capable of monophasic and charge-balanced biphasic, programmable stimulation are in development. To minimize scars from surgical placement, the single and dual channel designs are just over 10 mm in width, requiring a surgical incision of about ½ inch to place. Efficacy measurements are captured as (1) reliability of the connection to the Injectrode and (2) reliability of stimulation effects in chronic rodent testing.
Transitioning from acute Try Lead to chronic Receiver use case
Modeling the 90 day Try Lead phase, Injectrodes were fully implanted in rodent models on the left and right sciatic nerve. Powered by an EPG system to separately engage the left and right leg, limb joint angles were measured to assess chronic stimulation parameters for 1 to 5 months. Thereafter, an incision of approximately ½ inch was made to create a pocket in the subcutaneous tissue for the placement of the single-channel, biphasic Receiver model. Prior to implantation of the Receiver, the collector tail ends of both Injectrodes were externalized through the pocket without removing more than approximately ¼ of the length of each Injectrode, thereby leaving the stimulating tail end of each Injectrode on the respective sciatic nerve. Both Injectrodes were connected to the Receiver, followed by placement of the circuit into the pocket and closure of the incision.
Figure 3 Resonance-coupled receiver circuit model before dip-coating in thermoplastic polyurethane (TPU), and silicone coating in preparation for chronic preclinical model testing resembling the follow-up to the Try Lead phase that only had Injectrodes implanted on the left and right rodent sciatic.
Figure 4 left showcases the IPG receiver coil arrangement, featuring high-Q PCB coils designed for minimal cross-coupling between control channels. The right presents different functional implants under the IPG system, including single-channel, biphasic, and triple-channel IPGs, each capable of independent single-phase stimulation for multiple targets.
Figure 5 Internal driver in chronic preclinical testing, confirming long term stability and reliability
Figure 6 (a, b) Hind limb joint angles formed by the knee joint, ankle joint, and metatarsal head at rest. (c, d) Change in joint angles in response to 100Hz, 3.1V RFout, 300μs per phase biphasic stimulation of the IPG shown in Figure 1. Joint angles did not change in a different transmission coil orientation to the IPG coils.
Receiver circuit providing a test-bed for connecting to Try Lead Injectrodes
Neuronoff’s vision is to offer patients and physicians the option to connect an implanted driver circuit to an Injectrode after the Try Lead period of 90 days. Different connection designs have been developed and are being evaluated for their chronic long-term efficacy and stability. Neuronoff’s resonance-coupled Receiver circuit is enabling the initial chronic testing and subsequent evaluation of a “Handshake” adapter that allows the connection of a battery powered IPG to one or more Injectrodes.
Injectrode “Handshake” connector to battery-powered IPG
Neuronoff’s vision is to offer patients and physicians the option to connect an implanted driver circuit to an Injectrode after the Try Lead period of 90 days. Different connection designs have been developed and are being evaluated for their chronic long-term efficacy and stability. Neuronoff’s resonance-coupled Receiver circuit is enabling the initial chronic testing and subsequent evaluation of a “Handshake” adapter that allows the connection of a battery powered IPG to one or more Injectrodes.
Figure 7 Handshake adapter models with the ability to connect to 2 or 4 Injectrodes shown in preclinical testing.